Good news | The first in the country! Alzheimer's Disease Peripheral Blood Combined Test Kit Approved National Medical Device Registration Certificate
Saiji Biological A β 1-42/A β 1-40/T Tau/p Tau-181/ α- Synuclein Joint Detection Kit (Flow Fluorescence Luminescence Method) has been awarded the Medical Device Registration Certificate (In Vitro Diagnostic Reagent)! As the first joint testing reagent kit in the country to obtain a registration certificate, we are now recruiting agents from various regions across the country. We hope to work together to promote the development of clinical applications of flow cytometry technology and create a shared future for clinical flow cytometry!
Assist in early screening of elderly dementia
Grasp the golden window period and receive early diagnosis and treatment
Alzheimer's disease (AD), also known as senile dementia, is a neurodegenerative disease characterized by insidious onset and progressive deterioration of cognitive function, often accompanied by impaired daily living abilities and abnormal mental behavior. At present, the number of people affected globally exceeds 50 million, and it is expected to reach 150 million by 2050. The latest research points out that the prevalence of novel coronavirus has also greatly increased the risk factors of AD in the elderly. Therefore, early detection of AD biomarkers is crucial for delaying disease, altering disease progression, and even preventing disease through early intervention strategies.
The approved Alzheimer's disease peripheral blood combined testing project simultaneously tests "A" β 1-42, A β 1-40, T-Tau, p-Tau-181 and α- The levels of multiple biomarkers "synuclein" can help to comprehensively evaluate the protein deposition in the brain of the body in the early stage, comprehensively judge the cognitive state of the body, and provide important references for early screening and diagnosis and treatment of senile dementia

A β 1-42, A β 1-40, T-Tau, p-Tau-181 and α- Synuclein joint detection
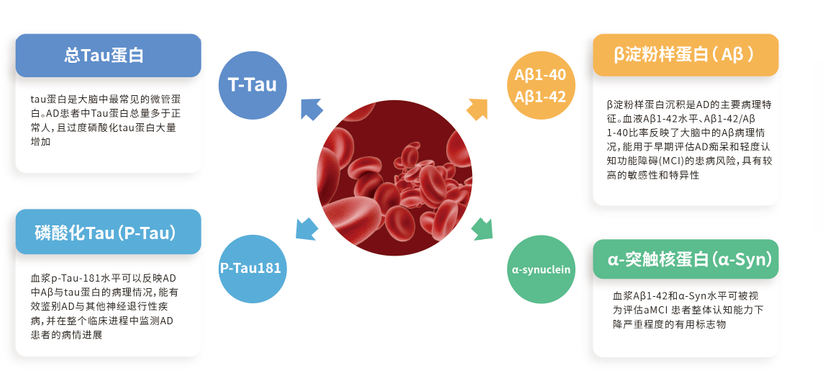
Product features
The combined detection of multiple peripheral blood biomarkers can provide more accurate prediction results;
Compared with invasive and expensive detection methods such as CSF and PET, the combined detection of peripheral blood biomarkers only requires one tube of blood, is easy to operate, has less trauma, and can be popularized in a large population;
Expected to provide more convenient, efficient, and accurate detection for the auxiliary diagnosis of AD
clinical application
Early screening and diagnosis of AD
The combined detection of peripheral blood biomarkers is expected to identify corresponding pathological changes 15-20 years before clinical symptoms appear, thereby assisting in early screening of AD and buying time for timely intervention of the disease.
Assessment related to AD treatment
After targeted drug therapy or other related treatments, combined detection of peripheral blood biomarkers can dynamically monitor the treatment effect and prognosis of AD patients.
Primary healthcare
The expert group suggests using blood tests as part of the diagnostic process for patients with cognitive symptoms in specialized memory clinics; At the same time, combined with health examinations, the elderly population can undergo regular annual screening and early intervention.













Please first Loginlater ~